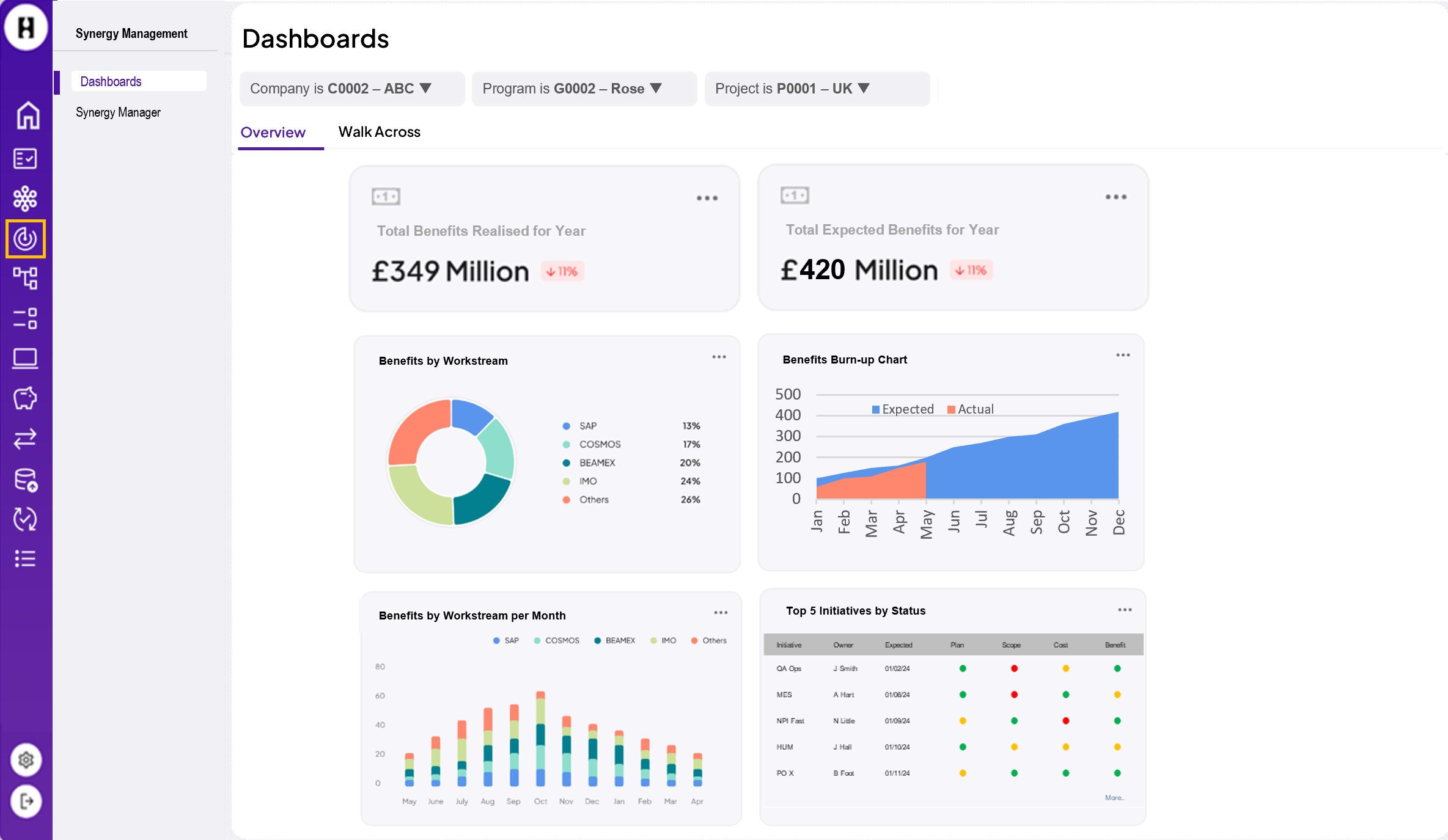
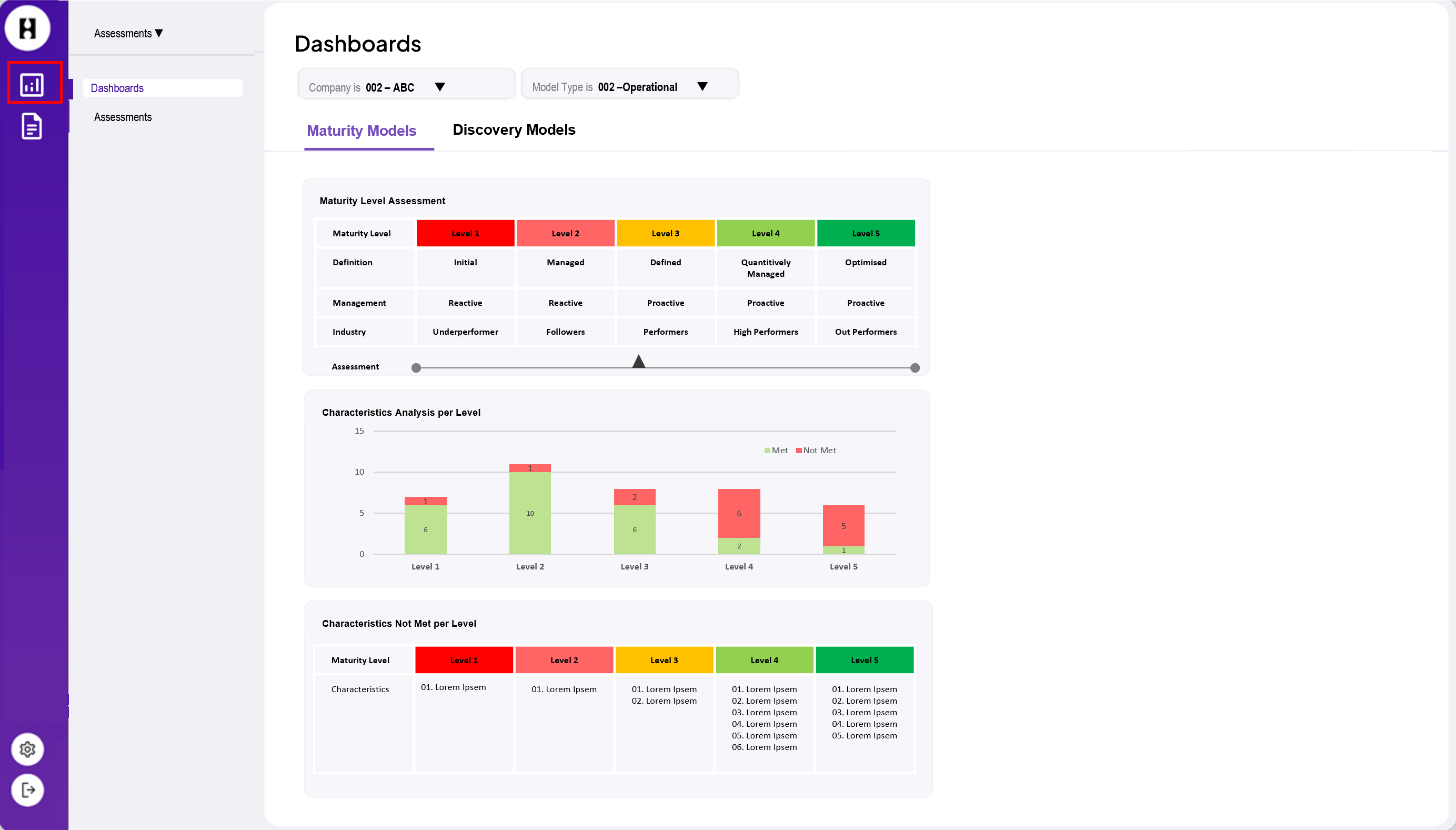
Connecting SAP HANA to Power BI: Why it matters and how to do it

Integrating SAP HANA with Power BI allows organisations to leverage SAP HANA’s robust data processing capabilities while taking advantage of Power BI’s user-friendly and advanced analytics features.
IDMP – Easing the burden of compliance
IDMP (Identification of Medicinal Product) helps to improve product and patient safety for medicinal products across a range of life sciences organisations, such as CROs, biopharma companies and global regulators.
Drug development with quantum computing

It is anticipated that quantum computing will be able to predict and simulate the structure, properties, and behaviour (or reactivity) of molecules more effectively than conventional computing can.
Explaining the drug production life-cycle … in 3 minutes

Back Explaining the drug production life-cycle … in 3 minutes Share: Related solution Business transformation We provide and deliver solutions to meet your goals. On time. On budget. The life cycle of a drug from its eureka moment to the bedside is a long and complex one and there’s a lot of written information available […]
SAP certification – why?

Continual professional development is just one way for us to push ourselves and, ultimately, our customers forwards.
Artificial Intelligence – the role of AI in early drug discovery

The latest advances in AI and Machine Learning (ML) play a major role in every sector of the pharmaceutical industry, from early drug discovery to manufacturing, by speeding up processes, reducing costs, and giving better direction to projects.
Our AI augmented future

Could AI really be the invention to end all human inventions?
Navigating the Brazilian SPED system with Thomson Reuters ONESOURCE

SPED has become the new fiscal compliance system in Brazil, and every company must submit their filings electronically at regular intervals to remain compliant.
Artificial Intelligence in healthcare – a new digital era
The healthcare sector is a challenging sector to be digitalised due to the mountain of information required to train the algorithm. Technological advances in this field have made great strides starting from video calls with doctors to computer algorithms for diagnosis.
Efficient warehouse operations in the life sciences industry

The processes followed in warehouse operations to manufacture and supply a finished product, are absolutely key to its successful rollout and distribution.